The mole
A mole of a substance is the amount that contains the same number of units as the number of carbon atoms in 12 grams of carbon-12. One mole is the Avogadro number of particles (atoms, molecules,ions or electrons) in a substance.
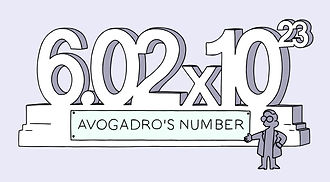
The Avogadro number
One mole of atoms contains 6.02x1023 atoms, no matter what elementit is. This is a very large number: it is 6 with 23 zeros after it. It is known as the Avogadro number.
For example:
Sodium is made of single sodium atoms. Iodine is made of iodine molecules. Its formula is
Its symbol is Na. Its Ar is 23. I2. Its Mr is 254.
This is 23grams of sodium. Its contains This is 254 grams of iodine. It contains 6.02x1023
6.02x1023 sodium atoms, or 1 mole of iodine molecules, or 1 mole of iodine molecules
sodium atoms.