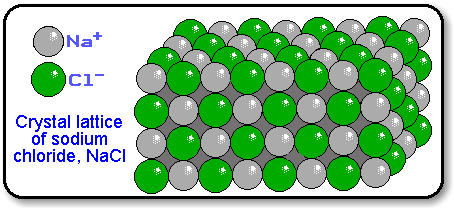
The formula of an ionic compound
From empirical to final formula
You saw in the last unit that the empirical formula shows the simplest ratio in which atoms combine.
The formula of an ionic compund is the same as its emprical formula.
The formula of a molecular compound
The molecular formula shows the actual numbers of atoms that combine to form a molecule.
The molecular formula is more useful than the empirical formula, because it gives you more information.
How to find the molecular formula
To find the molecular formula for an unknown compound, you need to know these:
- the relative molecular mass of the compund (Mr). This can be found using a mass spectrometer.
- its empirical formula. This is found by experiment.
- its empirical mass. This is the mass calculated using the empirical formula and Ar values.
Once you know those, you can work out the molecular formula by following these steps:
To find the molecular formula:
i Calculate Mr for the compound. This gives a number, n.
empirical mass
ii Multiply the numbers in the empirical formula by n.

Calculating the molecular formula
Example: A molecular compound has the empirical formula HO. Its relative molecular mass is 34. What is its molecular formula? (Ar: H=1, O=16).
For the empirical formula HO, the empirical mass = 17. But Mr = 34.
So Mr 34
empirical mass = 17 = 2
So the molecular formula is 2x HO, or H2O2.
So the compound is hydrogen peroxide.
Note how you write the 2 after the symbols, when you multiply.