Follow Us
Reminder: the total mass does not change
From the equation for a reaction, you can tell :
Calculations from equations, using the mole
For example :
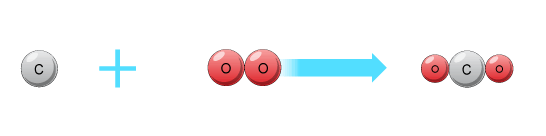
When Carbon burns in oxygen the reaction can be shown as
This equation tells you that :
1 carbon atom reacts with 1 molecule of Oxygen to give 1 molecule of carbon dioxide
Now suppose there is 1 mole of carbon atoms. Then we can say that :
1 mole of carbon atoms reacts with 1 mole of oxygen molecules to give 1 mole of carbon dioxide molecules
The Ar values are: C=12, O=16.
So the Mr values are: O2=32, CO2=(12+32) =44, and we can write:
12g of carbon reacts with 32g of oxygen to give 44g of carbon dioxide
Since substances always react in the same ratio, this also means that:
6g of carbon reacts with 16g of oxygen to give 22g of carbon
Calculating masses from equations
These are the steps to follow:
1. Write the balanced equation for the reaction.
2. Write down the Ar or Mr for each substance that takes part.
3. Using Ar or Mr, change the moles in the equation to grams
4. Once you know the theoretical masses from the equation, you can then find any actual mass.
Example: Hydrogen burns in oxygen to form water. What mass of oxygen needed for 1g of hydrogen, and what mass of water is obtained?
1. The equation for the reaction is: 2H2(g) + O2(g)---> 2H2O(g)
2. Ar: H= 1,O= 16. Mr: H2= 2, O2= 32, H2O= 18
3. So, for the equation, the amounts in grams are:
2H2(g) + O2(g) ---> 2H2O(g)
2x2g 32g 2x18g or
4g 32g 36g
4. But you start with only 1g of hydrogen, so the actual masses are:
1g 32/4g 36/4g or
1g 8g 9g
So 1g of hydrogen needs 8g of oxygen to burn, and gives 9g of water.
Working out equations, from masses.
Example: Iron reacts with a solution of copper(2) sulfate (CuSO4) to give copper and a solution of iron sulfate. The formula for the iron sulfate could be either FeSO4 or Fe2(SO4)3. 1.4g of iron gave 1.6g of copper. Write the correct equation for the reaction.
1. Ar: Fe= 56, Cu= 64.
2. Change the masses to moles of atoms:
1.4/56 moles of iron atoms gave 1.6/64 moles copper atoms, or
0.025 moles of iron atoms gave 0.025 moles of copper atoms, so
1 mole of iron atoms gave 1 mole of copper atoms.
3. So the equation for the reaction must be:
Fe + CuSO4 ----> Cu + FeSO4
4. Add the state symbols to complete it:
Fe(s) + CuSO4(aq) ----> Cu(s) + FeSO4(aq)
-how many moles of each substances take part
-how many grams of each substance take part
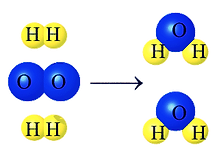
This is because no atoms have disappered. They have just been rearranged.